PUBLICATIONS
41. J. Mondal, P. Das, S. M. Husain* Isocitrate Dehydrogenase from Escherichia coli displays High Solvent Tolerance during NADPH generation Org. & Biomol. Chem. 2025, DOI: https://doi.org/10.1039/D5OB00917K

40. T. Manna, P. Das, A. Rabbani, F. Beyer, C. Merten, S. M. Husain* Biocatalytic Stereodivergent Synthesis of Substituted Tetrahydro-Benzo-(Oxa, Thia, and Di)-Azepines Chem. A Eur J. 2025, e202404537 DOI: https://doi.org/10.1002/chem.202404537

39. A. De, A. Shukla S. M. Husain* One-Pot Multienzyme Cascades for Stereodivergent Synthesis of Tetrahydroquinolines Angew Chem. Inter. Ed. 2024, DOI: https://onlinelibrary.wiley.com/doi/10.1002/ange.202411561

38. A. De, T. Manna, S. C. Debnath, S. M. Husain* Organophotoredox-catalyzed stereoselective reductive dimerization of chromone-2-carboxylic esters New Journal of Chemistry 2024, 48, 1902-1906. DOI: https://doi.org/10.1039/D3NJ05659G

37. A. Rajput, T. Manna, S. M. Husain* Anthrol reductases: discovery, role in biosynthesis and applications in natural product syntheses Natural Product Reports 2023, 40, 1672-1686. DOI: https://doi.org/10.1039/D3NP00027C

36. A. Rajput, T. Manna, A. Mondal, A. De, J. Mondal, S. M. Husain* Biocatalytic Access to Chiral Benzazepines Using Imine Reductases ACS Catalysis 2023, 13, 9, 6185–6194. DOI: https://doi.org/10.1021/acscatal.3c00146


35. A. Mondal, N. Saha, S. M. Husain* Concise chemoenzymatic total synthesis of (−)-rubroskyrin, (−)-deoxyrubroskyrin (−)-luteoskyrin, and (−)-deoxyluteoskyrin Tetrahedron Chem. 2022, 3, 100030. DOI: https://doi.org/10.1016/j.tchem.2022.100030
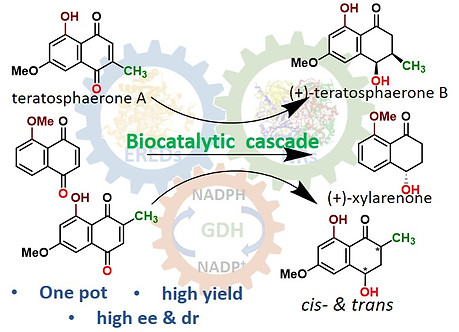
34. T. Manna, A. De, K. Nurjamal, S. M. Husain* Asymmetric synthesis of (+)-teratosphaerone B, its non-natural analogue and (+)-xylarenone using an ene- and naphthol reductase cascade Org. & Biomol. Chem. 2022, 20, 7410- 7414. DOI: https://doi.org/10.1039/D2OB01262F
33. A. De,+ N. Saha,+ T. Manna, V. Singh, S. M. Husain* Highly Efficient One-Pot Multienzyme Cascades for the Stereoselective Synthesis of Natural Naphthalenones ACS Catalysis 2022, 12, 19, 12179-12185. DOI: https://doi.org/10.1039/D2OB00401A +equal contributions

32. T. Manna, A. Rajput, N. Saha, A. Mondal, S. C. Debnath, S. M. Husain* Chemoenzymatic Total Synthesis of Nodulones C & D using a Naphthol Reductase of Magnaporthe grisea Org. & Biomol. Chem. 2022, 20, 3737 - 374. DOI: https://doi.org/10.1039/D2OB00401A First appeared on Chemarxiv 2021, DOI: https:10.26434/chemrxiv-2021-3hr0t

31. A. Rajput, A. De, A. Mondal, K. Das, B. Maity, S. M. Husain* A Biocatalytic Approach towards the Preparation of Natural Deoxyanthraquinones and their Impact on Cellular Viability New J. Chem 2022, 46, 3087-3090 DOI: https://doi.org/10.1039/D1NJ05513E

30. A. Rajput, A. Mondal, S. K. Pandey, S. M. Husain* Synthesis of rhein and diacerein: a chemoenzymatic approach using anthrol reductase of Talaromyces islandicus Org. & Biomol. Chem. 2022, 20, 358-361. DOI: https://doi.org/10.1039/D1OB02202D

29. T. Manna, A. Rajput, N. Saha, A. Mondal, S. M. Husain* Asymmetric Synthesis of Nodulones C & D by Chemoenzymatic Approach gives Insight into their Biosynthesis Chemarxiv 2021, DOI: https://chemrxiv.org/engage/chemrxiv/article-details/614980ad1df4a1749a76a858

28. S. K. Singh, A. Rajput, Arijit De, T. Chakraborti S. M. Husain* Promiscuity of an Unrelated Anthrol Reductase of Talaromyces islandicus WF-38-12 Cat. Sci. & Technology 2021, 11, 474-478. DOI: https://doi.org/10.1039/D0CY02148B

27. A. Modal, Arijit De, S. M. Husain* Synthesis of (‒)-Flavoskyrins by Catalyst Free Oxidation of (R)-Configured Dihydroanthracenones in Aqueous Media and its (Bio)synthetic Implications Organic Letters 2020, 22, 8511-8515. DOI: 10.1021/acs.orglett.0c03121

26. S. K. Singh, A. Mohammad, O. A. Alghamdi, S. M. Husain* New Approaches for targeting drug resistance through drug combination Book Chapter in Combination Therapy Against Multidrug Resistance 2020 Edited by M. Y. Wani & A. Ahmed Published by Elsevier (978-0-12-820576-1)

25. A. Mondal,+ S. K. Singh,+ N. Saha, S. M. Husain* Synthesis of Racemic Dihydroanthracen-1(2H)-ones using Sodium Borohydride in Water Eur J. Org. Chem. 2020, 2425-2430. DOI: 10.1002/ejoc.202000096 Very Important Paper

24. A. Mondal, S. K. Singh, T. Manna, S. M. Husain* Chemoenzymatic, Biomimetic Total Synthesis of (‒)-Rugulosin B, C and Rugulin Analogues and its Biosynthetic Implications ChemComm 2020, 56, 3337-3340. DOI: 10.1039/D0CC00406E

23. S. K. Singh,+ A. Mondal,+ N. Saha, S. M. Husain* Identification and Characterization of an Anthrol Reductase from Talaromyces islandicus (Penicillium islandicum) WF-38-12 Green Chemistry 2019, 21, 6954-6959. DOI: 10.1039/C9GC03072G

22. S. M. Husain,+ A. Präg,+A. Linnenbrink, A. Bechthold, M. Müller Insights into the Role of Ketoreductases in the Biosynthesis of Partially Reduced Bacterial Aromatic Polyketides ChemBioChem 2020, 21, 780-784. DOI: https://doi.org/10.1002/cbic.201900357

21. A. Mondal,+ N. Saha,+ A. Rajput, S. K. Singh, B. Roy, S. M. Husain* Chemoenzymatic Reduction of Citreorosein and its Implications for Aloe-Emodin and Rugulosin C (Bio)synthesis Org. Biomol. Chem. 2019, 17, 8711-8715. DOI: 10.1039/C9OB01690B

20. N. Saha, M. Müller, S. M. Husain* Asymmetric Synthesis of Natural cis-Dihydroarenediols Using Tetrahydroxynaphthalene Reductase and Its Biosynthetic Implications Org. Lett. 2019, 21, 2204-2208.

19. S. K. Singh, S. M. Husain* A Redox-based Superoxide Generation System using Quinone/Quinone Reductase
ChemBioChem 2018, 19, 1657-1663.

18. N. Saha+, A. Mondal+, K. Witte, S. K. Singh, M. Müller, S. M. Husain* Monomeric Dihydroanthaquinones: A Chemoenzymatic Approach and its (Bio)synthetic Implications for Bisnathraquinones Chem. Eur. J. 2018, 24, 1283-1286. +equal contribution

17. S. M. Husain, M. Müller* Fungal Dihydroxynaphthalene-Melanin: Diversity-Oriented Biosynthesis through Enzymatic and Nonenzymatic Transformations Synlett 2017, 28, 2360-2372.

16. L. Fürtges, D. Conradt, M. A. Schaetzle, S. K. Singh, N. Kraševec, T. L. Rižner, M. Müller,* S. M. Husain* Phylogenetic Studies, Gene Cluster Analysis, and Enzymatic Reaction Support Anthrahydroquinone Reduction as the Physiological Function of Fungal 17beta-Hydroxysteroid Dehydrogenase ChemBioChem 2016, 18, 77-80. [Link]

15. J. Haas, M. A. Schätzle, S. M. Husain, J. Schulz-Fincke, M. Jung, W. Hummel, M. Müller, S. Lüdeke A quinone mediator drives oxidations catalysed by alcohol dehydrogenase-containing cell lysates Chem. Comm. 2016, 52, 5198-5201.

14. M. A. Schätzle, S.M. Husain, M. Müller Tetrahydroxynaphthalene Reductase – Catalytic Properties of an Enzyme Involved in Reductive Asymmetric Naphthol Dearomatization Book Chapter in Practical Methods for Biocatalysis and Biotransformations-3- 2016 Edited by Whitall, Sutton, and Kroutil

13. D. Conradt, M. A. Schätzle, S.M. Husain, M. Müller Diversity in Reduction with Short-Chain Dehydrogenases: Tetrahydroxynaphthalene Reductase, Trihydroxynaphthalene Reductase, and Glucose Dehydrogenase Chem.Cat. Chem. 2015, 7, 3116-3120.

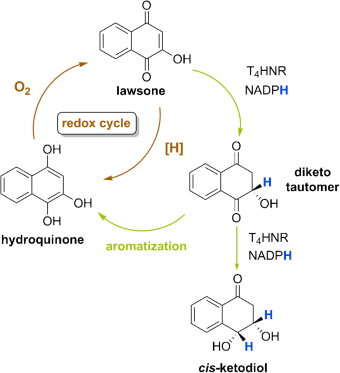
12. S.M. Husain, M. A. Schätzle, S. Lüdeke, M. Müller Unprecedented Role of Hydronaphthoquinone Tautomers in Biosynthesis Angew. Chem. Int. Ed. 2014, 53, 9806-9811.
Mentored Publications
11. M. A. Schätzle, S. M. Husain, S. Ferlaino, M. Müller Tautomers of Anthrahydroquinones: Enzymatic Reduction and Implications for Chrysophanol, Monodictyphenone, and Related Xanthone Biosyntheses J. Am. Chem. Soc. 2012, 134, 14742-14745. [PDF] [Supporting info]
10. S. M. Husain, M. A. Schätzle, C. Röhr, S. Lüdeke, M. Müller Biomimetic Asymmetric Synthesis of (R)-GTRI-02 and (3S,4R)-3,4-Dihydroxy-3,4-dihydronaphthalene-1(2H)-ones Org. Lett. 2012, 14, 3600-3603. [PDF] [Supporting info]
9. J. Kulig, R. C. Simon, C. A. Rose, S. M. Husain, M. Häckh, S. Lüdeke, K. Zeitler, W. Kroutil, M. Pohl, D. Rother Stereoselective synthesis of bulky 1,2-diols with alcohol dehydrogenases Catal. Sci. Technol. 2012, 2, 1580-1589.[PDF] [Supporting info]
8. M. A. Schätzle, S. Flemming, S.M. Husain, M. Richter, S. Günther, M. Müller Tetrahydroxynaphthalene Reductase – Catalytic Properties of an Enzyme involved in Reductive Asymmetric Naphthol Dearomatization Angew. Chem. Int. Ed. 2012, 51, 2643-2646. [PDF] [Supporting info]
7. S. M. Husain,T. Stillger, P. Dünkelmann, M. Lödige, L. Walter, E. Breitling, M. Pohl, M. Bürchner,I. Krossing, M. Müller, D. Romano,F. Molinari Stereoselective reduction of 2-hydroxy ketones towards syn– and anti-1,2-Diols Adv. Synth. Catal. 2011, 353, 2359-2362. [PDF] [Supporting info]
From Ph.D.
6. S. Sarkar, S. M. Husain, D. Schepmann, R. Fröhlich, B. Wünsch Microwave assisted synthesis of 3-benzazepin-2-ones as building blocks for 2,3-disubstituted tetrahydro-3-benzazepines Tetrahedron 2012, 68, 2687-2695.[PDF]
5. S. M. Husain, R. Fröhlich, D. Schepmann, B. Wünsch Enantioselective Synthesis of a 2,2-Disubstituted Tetrahydro-3-benzazepine as Novel Receptor Antagonist Z. Naturforsch. 2010, 65b, 191-196.
[PDF]
4. Asymmetric Synthesis and σ receptor affinity of enantiomerically pure 1,4-disubstituted-tetrahydro-1H-3-benzazepines
S. M. Husain, M. T. Heim, D. Schepmann, B, Wünsch, Tetrahedron: Asymmetry, 2009, 20, 1383-1392.
[PDF]
3. S.M. Husain, R. Fröhlich, D. Schepmann, B. Wünsch Asymmetric Synthesis of Enantiomerically pure 2-Substituted-3-benzazepines and Their Affinity to σ1 Receptors J. Org. Chem. 2009, 74, 2788-2793.
[PDF] [Supporting Info]
2. S. M. Husain, R. Fröhlich, B. Wünsch A very short asymmetric synthesis of enantiomerically pure methyl substituted tetrahydro-3-benzazepines Tetrahedron: Asymmetry, 2008, 19, 1613-1616.
[PDF]
1. S. M. Husain, B. Wünsch, Synthesis of Phenylacetic Acids with 2-Oxoalkyl Substituents in ortho-Position from o-Phenylenediacetic Acid Synthesis, 2008, 17, 2729-2732.
.png)
